Even as the apex court pulled up Patanjali Ayurved for misleading ads, there are allegations of pharma companies giving Rs 1,000 crore to political parties through electoral bonds. Are these “underhand deals” now claiming lives?
By Sanjay Raman Sinha
Patanjali Ayurved’s efforts to peddle its questionable drugs in the marketplace and the silence of the government is just the tip of the iceberg, pointing to the existence of a larger cabal that is pushing spurious medical products and drugs to gullible customers.
With the electoral bonds issue cropping up, the sinister nexus between the central and state governments and pharma companies has become evident. Two trends are discernable in the electoral funding pattern of pharma firms. First, many pharma companies who donated large sums via electoral bonds were under the scanner of the Enforcement Directorate and Income Tax department. Huge amounts of bonds were bought by these companies following searches and raids. Secondly, at least 22 of the 35 pharma firms donated Rs 945 crore worth of electoral bonds to parties holding power in states where the firms have manufacturing units. This is because the power lies with the state government to take action against drug companies’ manufacturing units. At least 11 political parties took money from pharma companies and most were regional outfits. In many instances, drug companies donated money to the BJP after central agencies initiated a probe into alleged tax evasion or other financial crimes like money laundering (see box: Pharma quid pro quo).
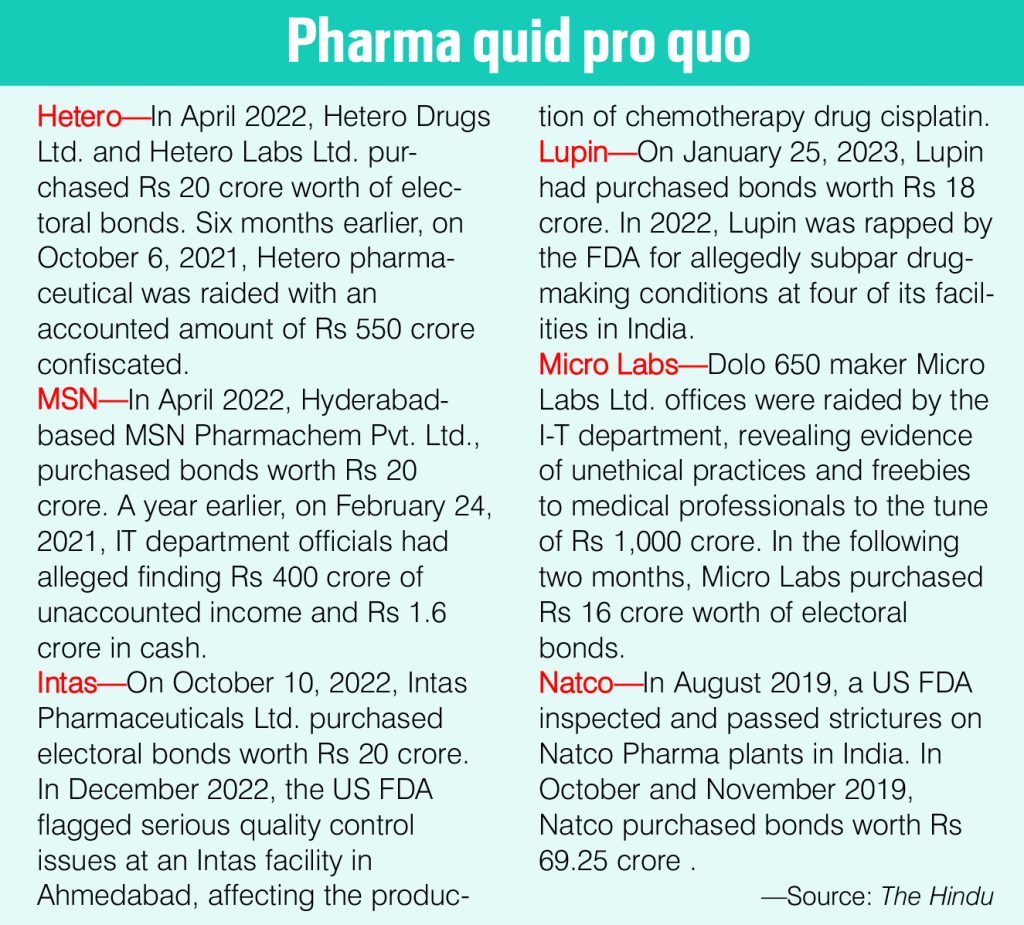
The quid pro quo, often insinuated in the Supreme Court’s hearings of electoral bonds, is corroborated by the fundings by pharma companies. The most recent example of big pharma malfeasance is Patanjali Ayurved.
Recently, Patanjali Ayurved faced the heat in the Supreme Court as a two-judge bench took to task the company founder, yoga guru Ramdev, and its managing director Acharya Balakrishna for their misdemeanours and brazen behaviour. Justices Hima Kohli and Ahsanuddin Amanullah took strong exception to Patanjali for the breach of undertaking it had given to the Court.
On February 27, the Court had issued a contempt notice to Patanjali Ayurved in response to the company’s continued publication of misleading advertisements claiming to offer permanent cures for various diseases. In November 2023, the company had given an assurance to the Court “to not release any statements that made unsubstantiated claims about medicinal efficacy or criticise any system of medicine”. However, Patanjali Ayurved kept on with its misleading advertisements.
Taking note of this insolent intransigence, the bench lambasted the company for misleading the nation and cocking a snook at the Court. The bench warned the company and also upbraided the central government for failing to take any action against misleading advertisements despite being aware of them.
The bench told Solicitor General Tushar Mehta to file a detailed affidavit to “dispel the impression” that the government machinery, both at the central and state levels, was complicit with Patanjali. During the hearing, Justice Kohli stressed: “What you have to dispel is that you and the state government are not complicit in this whole activity…. Disparaging other fields of medicine in the manner that has been done by the proposed contemnors is most unacceptable. We are assuming even if you did not put it in the public domain, at least you told them that it is nothing more than a supplementary, do not tom-tom it as a cure. Still, you chose to keep your eyes shut. We are wondering why Union of India did it.” And in a dramatic moment, Ramdev’s lawyer apologised to the Court with folded hands.
Even as the Supreme Court ticked off the government for turning a Nelson’s Eye to Patanjali Ayurved’s misdeeds, the Congress alleged that seven of the 35 pharmaceutical companies which have contributed Rs 1,000 crore to political parties through electoral bonds were being investigated for manufacturing poor quality drugs such as cough syrups and Remdesivir. Congress general secretary Jairam Ramesh alleged “underhand deals” between the government and the pharmaceutical companies so that they can carry out their businesses after giving donations to the ruling party. “Chanda Do, Dhanda Lo” was his refrain.
This is not the first time that Patanjali Ayurved has flouted norms to mint money in the pharma marketplace. In August 2022, the Indian Medical Association (IMA) took legal action in response to a Patanjali advertisement titled, “Misconceptions spread by Allopathy: Save yourself and the country from the misconceptions spread by pharma and medical industry”. The twin charges propagated misinformation to denigrate Allopathy and exaggerated claims about the efficacy of its own drugs.
According to the IMA, these statements violated both the Drugs and Other Magical Remedies Act, 1954 (DOMA) and the Consumer Protection Act, 2019 (CPA).
Dr Punita Hasija, past president, IMA Haryana, told India Legal: “Misleading advertisements are not a good thing. It makes people buy medicines which may not be conducive to health and create serious health problems. The Indian Medical Association has taken a very strong stand on the issue and we are thankful to the Supreme Court for dealing with the issue strictly.” (See box on Patanjali’s faulty products.)
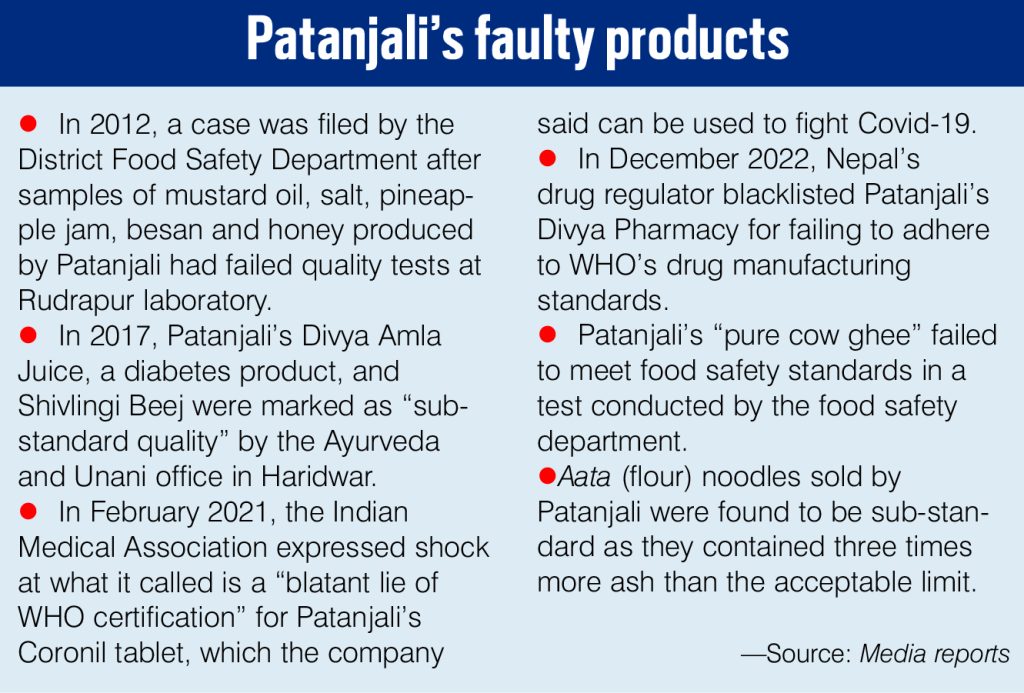
Misleading health advertisements are the bane of the medical and pharma industry and the exaggerated health benefits are often not backed by medical research. The ads often use famous personalities to provide testimonials about the advertised product, thus easily fooling people, often with harmful results. Teleshopping sites and TV capsules abound with such exaggerated claims for medical products and services.
In 2019, 61 companies faced probe by the industry watchdog Advertising Standards Council of India (ASCI) for misleading health ads and violation of advertising guidelines. The ASCI found violation of norms by companies dealing with health products like hair oil, cough syrups, advanced bariatric and robotic surgeries, among others. Many products were promoted by well-known companies. The ASCI website abounds with such cases.
Dr Naresh Chawla, vice president of the Delhi Medical Council, told India Legal: “Misleading the innocent and gullible public by advertisements is a very serious issue. Patanjali of Baba Ramdev is a recent example. The Supreme Court has taken a strong stand and will penalise. The government should not only enact stringent laws, but implement them religiously.”
According to an Economic Times report of June 6, 2013: “A maximum of 31 complains were upheld in the health and personal care sector, where big firms such as Dabur, Procter & Gamble Hygiene & Health Care, Hindustan Unilever and Johnson & Johnson were found to be violating ASCI code.”

The ASCI, unfortunately, lacks teeth, and the regulatory framework to act against pharma crimes is woefully inadequate. The last two years have seen a barrage of cases where generic cough syrups have killed children, eye drops have caused blindness and cancer drugs found contaminated.
In 2022, 66 children in Gambia died a painful death after they were given Indian-made cough syrup. The syrup was manufactured by India’s Maiden Pharmaceuticals and exported to the African country. In 2019, 17 children died in Jammu and Kashmir after consuming a syrup made by a company called Digital Vision. The violations continue unabated.
India produces 20% of the world’s generic medicine, thereby getting the mantle of the biggest manufacturer. The nation boasts of a $50 billion drug-manufacturing industry which exports medicines to over 200 nations and makes 60% of all vaccines. However, the burgeoning Indian pharma industry also carries within its womb the seeds of deceit and devastation. Rogue medical firms play the game of death with reckless and unbridled abandon as grey areas in regulation make them operate with impunity and escape punishment. As per 2018 figures of the Central Drug Standard Control Organisation, about 4.5% of all generic drugs in the Indian market are substandard.
The risks of counterfeit drugs have cross-border ramifications as well. Apart from the Gambian tragedy in 2021, fake vials of remdesivir, an antiviral drug used to treat Covid, were sold in bulk at shockingly inflated prices and were even exported. In 2013, Ranbaxy Laboratories pleaded guilty in a US court to felony charges over the manufacture and distribution of adulterated drugs made in its manufacturing facilities in India. In 2016, two Indian pharmaceutical companies were charged with exporting counterfeit diabetes drugs. From 2022 till last year, Indian drug makers were issued nine US Food and Drug Administration warning letters. Checks at manufacturing units have been abysmally low and have been red flagged by the American body.
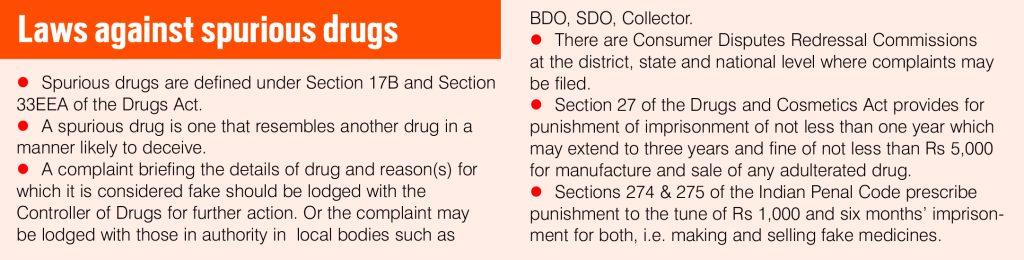
The right to health is a corollary to the right to life and guaranteed to citizens by the Constitution. Spurious drugs are drug formulations which conceal the true identity of the product and are sold resembling a popular brand. Drug regulation is governed by an outdated Drugs and Cosmetics Act. Despite modifications in the law, it is still ill-equipped to regulate a complex market.
Dr Punita Hasija said: “Quality control of drugs is the domain and responsibility of drug controllers. In every district, there are drug inspectors who inspect pharmacies and drug factories and ensure that proper guidelines are being adhered to for the safety and efficacy of medical products. These are life threatening practices and should definitely be curbed. IMA stands by the government for strict action against the wrongdoers. IMA doesn’t have penal powers, and the laws are not strong enough to deter wrongdoing. Hardly anyone gets punished.”
Last year, the central government launched a nationwide move against firms making substandard drugs and cancelled the manufacturing licenses of 18 pharmaceutical companies. Despite the penal action, the spurious drug market is booming and misleading medical advertisements add to the peril.
The drug business works with its lever-system of “incentives” or gifts to doctors. They are plied with freebies for prescribing preferred drugs. The case involving promotion and prescription of Dolo-650 mg tablets has gone to the court. Dolo-650 was the most commonly used treatment for Covid-19. The Federation of Medical & Sales Representatives Association of India (FMRAI) had accused the manufacturer of giving doctors gifts worth Rs 1,000 crore to get them to prescribe Dolo-650 as medication.
The petition of FMRAI said that the top seven pharma companies together had spent Rs 34,187 crore in marketing over eight years. These constitute 20% of the cost of drugs and include direct and indirect benefits to doctors such as gifts, entertainment, trips and hospitality.
It must be noted that the Supreme Court had ruled that pharma companies are not entitled to claim tax exemption on the expenditure incurred for giving incentives to doctors to promote their medical products. The whole business of gifts can turn unsalutary if unreliable products are pushed at the cost of reliable ones.
The Planning Commission has been quoted as saying that pharmaceutical marketing and aggressive promotion contributes to the irrational use of drugs and therefore, there is a need for a compulsory code to identify and penalise unethical promotion by these companies.
Drug pricing has been a sensitive issue with profiteering hitting patients. Healthcare capitalism has been at the forefront of jacked up prices.
As per media reports, for each dose sold to private hospitals, Serum made profits up to 2,000% and Bharat Bio Tech up to 4,000%. A committee was formed way back in 2015 to control drug prices, but the recommendations are yet to see the light of day. The government is yet to formulate a comprehensive policy on the trade margin of expensive medicines.
As the pharma buccaneers make hay while the sun shines, the dire need to reform the healthcare regime is acutely being felt. Watchdog bodies lack teeth, regulations have grey areas and the industry has no penchant for self-regulation. In such a scenario, as the courts wield the stick, it becomes incumbent for the government to proactively initiate structural and normative changes to ensure a safe and secure healthcare ecosystem.
It is also necessary that the government clears the smokescreen (as demanded by the Supreme Court) and cuts any umbilical cord that binds it to nefarious pharma companies and takes strict action against culpable companies.


